
Parents everywhere can breathe a bit easier this fall, knowing the Food and Drug Administration (FDA) has approved a long-lasting drug to protect children under age two from Respiratory Syncytial Virus (RSV). AstraZeneca is selling the new drug under the brand Beyfortus. It will contain an antibody capable of helping our youngest fight the potentially deadly disease. Considering how quickly cases of RSV can land young children in the hospital, this news is coming at the right time.
The News Was Recently Announced
The Associated Press (AP) shared snippets from the Director of the FDA’s Office of Infectious Diseases statement about the approval. It cited the importance of developing drugs that keep babies and toddlers out of the emergency room during cold and flu season.
“Today’s approval addresses the great need for products to help reduce the impact of RSV disease on children, families and the health care system,” the statement from Dr. John Farley read.
RSV Can Be Deadly for the Very Young and Very Old

Hearing the doctor say “RSV” can send chills down a parent’s spine. While most people with RSV only experience cold-like symptoms, high-risk individuals can experience life-threatening complications. These include “bronchiolitis (inflammation of the small airways in the lung)” and “pneumonia (infection of the lungs),” according to the Centers for Disease Control and Prevention (CD).1
Those complications can progress quickly, sending scared parents to the ER with their wheezing babies year after year.1
Cases Have Been Climbing Recently
In 2022, cases of RSV skyrocketed, with one doctor telling the AP that children’s hospitals in his area saw a 30 percent increase in patients, thanks to what he called a “viral jambalaya” that included a bump in RSV infections.
An estimated 58,000 children under age 5 are hospitalized yearly due to RSV in the United States.1 Sadly, hundreds of those children never make it home and die of complications from the illness, according to AP.
This new drug from Beyfortus hopes to change all that.
Studies Show the Drug Has Been Successful in Clinical Trials

It looks like the product has had good results. Beyfortus submitted three studies to FDA officials showing the drug reduced the risk of infection between 70 and 75 percent in the target demographic.
While the ultimate success range seems to lie in that danger zone for children under two, the CDC will still need to meet in August to decide its official approval guidelines, including the ages for which it will approve the drug.
This Antibody Treatment Isn’t Anything New
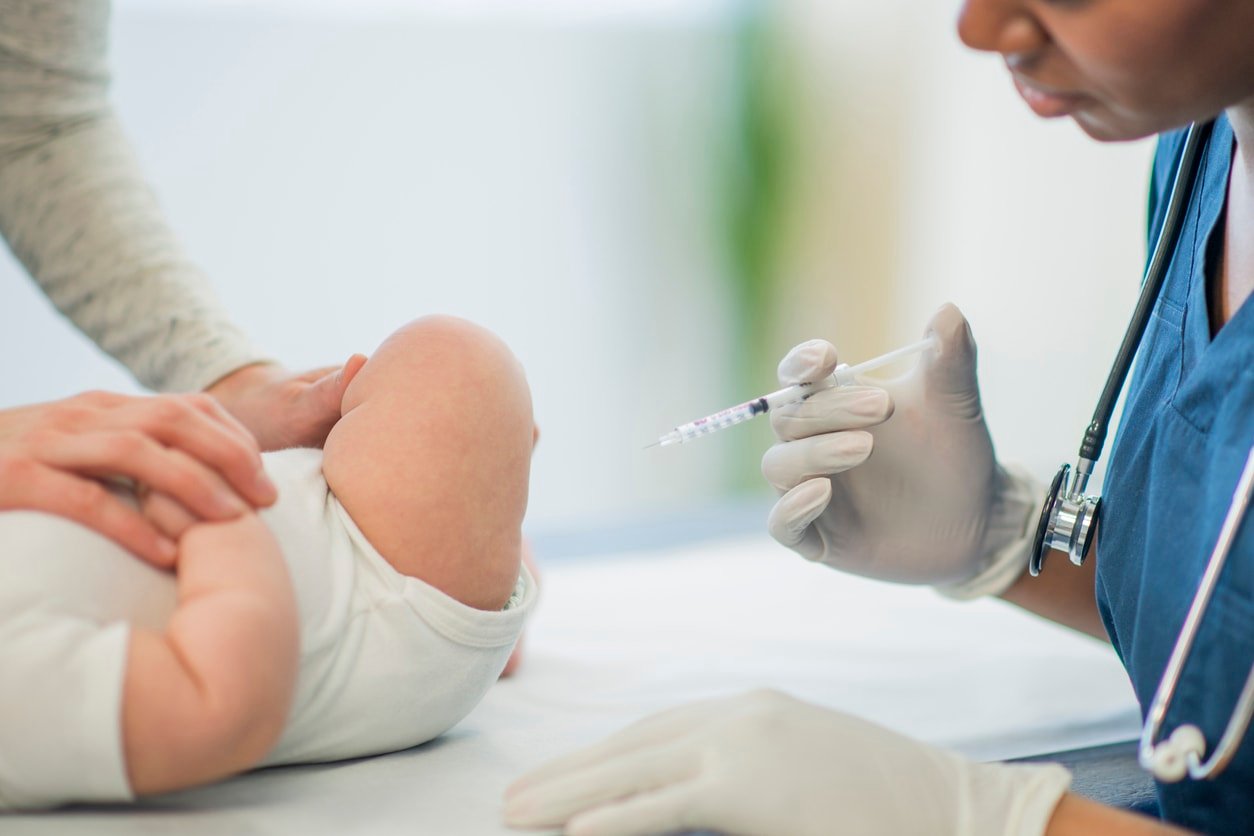
This is the first time U.S. doctors will be able to recommend this option. However, Canada, Europe, and the U.K. have approved Beyfortus for a while now.
A similar drug has been approved for use in high-risk babies for over 20 years. But this version requires monthly injections, whereas the new Beyfortus option appears to require annual injections.
This should be comforting news for new parents worried about this upcoming cold and flu season. Especially parents with high-risk or older children at school who may bring germs home to their little brothers or sisters. We know we’ll sleep better knowing our most vulnerable populations may have access to these drugs soon.
from Baby Chick https://ift.tt/GVQJN2s
via IFTTT


0 Comments
Please ,
Don't enter span link ...